- europages
- >
- ENTREPRISES - FOURNISSEURS - PRESTATAIRES
- >
- recherche scientifique - instituts et laboratoires
Résultats pour
Recherche scientifique - instituts et laboratoires - Import export

PROGENUS
Belgique
Contribuer au bien-être animal est un des engagements de Progenus. Nous avons à cœur de vous recommander les analyses génétiques propre à la race de votre animal. Il vous est également possible, via un profil ADN, de certifier son identité ainsi que sa filiation. Toutes ces analyses suivent la norme ISO17025 pour laquelle nous sommes accrédités. Nous sommes également certifiés ISAG 2006 avec un résultat au rings test en catégorie 1 (plus de 99% de fiabilité). En plus des maladies, vous pouvez effectuer d’autres tests afin de déterminer des marqueurs tels que la couleur de robe, la longueur des poils, la queue courte… Nous veillons au développement permanent de notre offre de tests en suivant quotidiennement les avancées du monde scientifique ou en y participant nous-même via notre département de Recherche. Vous avez besoin d’un test en particulier ou vous souhaitez avoir notre avis scientifique, nous sommes à votre écoute pour répondre à toutes vos questions.
Demander un devis
PROGENUS
Belgique
Nos services pour la recherche : - Services liés à l’ADN Développement de tests de PCR en temps réel, en simplex ou multiplex. Développement de méthodes d’extraction d’ADN pour tout type de matrices. Production de kits PCR (en tubes ou en plaques préremplies) selon la norme ISO13485. Développement de tests de LAMP (amplification ADN isotherme). Développement de PCR pour des applications de séquençage (Sanger et next generation sequencing NGS). Développement de tests d’analyse de fragments (analyse de fingerprint). - Services liés aux protéines Développement de tests ELISA. - Services liés aux pathogènes Développement de méthodes PCR pour la détection de virus et bactéries. Développement de lyses rapides sur bactéries et virus.
Demander un devis
DELPHI GENETICS
Belgique
GMP grade plasmid DNA production is based on robust, single-use, TSE-BSE-free methods and occurs in our GMP suite, at a maximum scale of 50L. Delphi Genetics offers one-stop-shop CDMO service for plasmid DNA GMP supply, including: GMP compliant Process and QC Development GMP E.coli cell banking (Master and Working Cell Banks) GMP DNA Manufacturing using TSE-BSE free and Single-use process, based on QP approved Master Batch Record QC and Release by Qualified Person (QP) Full Quality Control complying with the European pharmacopea 5.14 will be performed: Content and purity by UV measurement (260/280 nm) Visual inspection Identity by restriction and DNA sequencing (full plasmid sequencing) Safety tests: Endotoxins, Bioburden/Sterility and Mycoplasma Residual RNA content Residual host protein Residual host genomic by Q-QPCR pH Topology Other tests can be performed on request. QP Released GMP Plasmid DNA is provided with TSE-BSE-free certificate and Certificate of Analysis.
Demander un devis
DELPHI GENETICS
Belgique
Delphi Genetics provides customized plasmid DNA production services, from milligram to gram scale, for your R&D and clinical activities. We take care of your plasmid DNA material for viral vectors production and naked DNA-based gene therapy. Our service includes the supply of: R&D grade for non-clinical use, support for lead identification, clone screening, STABY evaluation. High Quality and GMP grade plasmid DNA for viral and non-viral (pre-)clinical activities.
Demander un devis
DELPHI GENETICS
Belgique
For manufacturing recombinant proteins or DNA, plasmid instability is a significant concern. Typical biomanufacturing processes in prokaryotes require the use of bacterial plasmids as vectors carrying the gene of interest to be over-expressed. It has been demonstrated that the growth of plasmid bearing cells is significantly reduced relative to plasmid free hosts, simply because protein production represents a significant burden on cellular metabolism. This is of significant concern in the Biopharmaceutical Industry as both the yield and the production reproducibility of recombinant molecules are significantly lowered by that fact. Antibiotic resistance genes are the most common selectable markers used in fermentation processes to avoid plasmid free cells to overgrow the culture. However antibiotics are expensive compounds and they (or their degradation products) can contaminate the biomass or production product.
Demander un devisVous vendez ou fabriquez des produits similaires ?
Inscrivez-vous sur europages et référencez vos produits

DELPHI GENETICS
Belgique
In order to maximize protein expression, the Staby® system was combined to the proven T7 polymerase technology for the over-expression of recombinant proteins: the gene of interest is under the control of the T7 promoter. This combination is called StabyExpress®. The pStaby1 plasmid contains the gene encoding the antidote protein (ccdA) which counteracts the action of the ccdB protein. The SE1 strain, in which the ccdB selection gene is inserted in the chromosome in order to stabilize the pStaby1 plasmid has been engineered by Delphi Genetics as a dedicated bacterial strain for protein expression and is derived from BL21(DE3). Consequently, IPTG has to be used to induce protein expression, an alternative strategy being the use of an auto-inducible medium (such as Delphi Genetics' Staby®Switch medium). Staby®Express ensures that only plasmid-bearing clones can grow whereas every clone losing the plasmid will inevitably die.
Demander un devis
DELPHI GENETICS
Belgique
With thousands of DNA vectors designed and constructed, Delphi Genetics is a Partner of choice to generate your plasmid of interest. Size does not matter, that's the strategy of cloning that drives the success of a plasmid construction. We perform the construction of your vector from strategy design to final plasmid production. We work with standard plasmid DNA as well as with large constructs, such as BAC and Cosmid. Our plasmid DNA construction service includes: Sequences analysis Design and strategy Gene Synthesis Cloning of PCR fragment DNA fragment assembling Site directed mutagenesis DNA sequencing Plasmid productionA new hope for Covid-19 treatment Delphi Genetics more than triples its production capacity and receives GMP certification. New Plasmid DNA production capacities now immediately available. Delphi to attend Phacilitate Leaders World in Miami, Jan 21-24 Delphi Genetics significantly expands its capacities for cGMP plasmid DNA GMP Certification for Plasmid DNA
Demander un devis
DELPHI GENETICS
Belgique
Delphi Genetics offers the benefits of its proprietary Staby® technology through partnering opportunities. We intend to build high-value strategic collaborations for exploring the benefits of the Staby® technology in various DNA & Protein bioproduction settings. Partnering is a key component of our service delivery strategy. Delphi Genetics is open to consider any collaborative projects at all stages of development from R&D to the commercialization of your bioproduct. We seek collaborative partnerships with Biotech & Pharma companies that could enable us to contribute to your development on either a risk-sharing or out-licensing basis. In order to consolidate a long term relationship with its partners, Delphi Genetics is currently developing a brand new GMP production facility. This mid-scale capacity facility will join technology and flexibility to provide plasmid DNA and protein with high value for your clinical phases.
Demander un devis
DELPHI GENETICS
Belgique
All E. coli strains can be adapted to the Staby® technology. This adaptation is linked to the insertion into the bacterial chromosome of the CcdB toxic gene. Based on our strong knowledge of E. coli genetic and our know-how in genetic engineering we can easily adapt the strain of your choice and genetically modify it for the Staby® technology. On the same basis, we can genetically modify the strain of your choice to introduce mutation, deletion or to restore activity of certain gene. We can also introduce new gene to give new characteristic to your strain. One example would be to allow your strain of interest to grow and use new or unusual substract. All these modifications are finalized without any genetic trace
Demander un devis
DELPHI GENETICS
Belgique
We are continuously improving and developing our Staby® technology to provide new adaptation proposing the best solution to our Customer. The Staby® technology has been adaptated in different fields and can be proposed on a tailor made solution basis or on a service basis. The Staby® technology applications actually available are the following. Feel free to contact us for other request
Demander un devis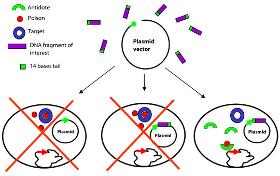
DELPHI GENETICS
Belgique
Traditionally clone selection was based on the disruption of a genetic marker (e.g. blue and white screening based on the disruption of the LacZ gene), plasmid retention was based on antibiotic resistance genes. The development of vectors employing selection modules circumvents these issues and permits growth of bacteria harbouring insert-bearing plasmids only. Typically, the vectors used in these systems express a gene product that is lethal to certain bacterial hosts. The lethal gene is inactivated by insertion of a segment of foreign DNA and therefore, toxicity is relieved. The most efficient technical solution remains the killing of bacteria harbouring an insertless vector, or the selection of bacteria harbouring the recombinant vector, the so-called positive selection. The ccdB technology developed by Delphi Genetics has proven to be a successful approach for constructing positive selection vectors
Demander un devis
DELPHI GENETICS
Belgique
Because the stabilization relies on the presence of only two genetic elements, one on the plasmid the other in the expression strain, the stabilization technology is readily adaptable into any existing E.Coli based expression platform. Delphi Genetics can customize any vectors and strains by adding the stabilization cassette into the vector and the selection gene into the chromosome of the chosen expression strain. Furthermore Delphi Genetics has developed a comprehensive licensing policy for those who would wish to integrate the stabilization system into an existing platform.
Demander un devis
DELPHI GENETICS
Belgique
Modern DNA engineering requires efficient and robust molecular tools. The Staby® Operating System, developed by Delphi Genetics is an original set of bacterial strains and vectors integrating unique patented technologies. The use of these products and technologies allows researchers to reach their goal faster and more efficiently. These technologies can be used in a project of any scale (from laboratory scale to industrial processes). The Staby® Operating system is very flexible and its elements can be integrated into your favorite tools, allowing you to add the power of the Delphi-Genetics technologies into your own platform.
Demander un devis
DELPHI GENETICS
Belgique
R&D plasmid DNA will be produced in shake flask or in fermentor (up to 8L), in our R&D facility, and purified by classical method. R&D plasmids are provided Endotoxin-free.
Demander un devis
DELPHI GENETICS
Belgique
High-Quality grade plasmid DNA production is based on robust, single-use, TSE-BSE-free methods and occurs in our GMP suite, at a maximum scale of 50L, after Delphi's process performance assessment. Full Quality Control complying with the European pharmacopea 5.14 will be performed: Content and purity by UV measurement (260/280 nm) Visual inspection Identity by restriction and DNA sequencing (full plasmid sequencing) Safety tests: Endotoxins, Bioburden/Sterility and Mycoplasma Residual RNA content Residual host protein Residual host genomic by Q-QPCR pH Topology Other tests can be performed on request. High quality Plasmid DNA is provided with TSE-BSE-free certificate and Certificate of Analysis.
Demander un devisRésultats pour
Recherche scientifique - instituts et laboratoires - Import exportNombre de résultats
16 ProduitsType d'entreprise