- europages
- >
- ENTREPRISES - FOURNISSEURS - PRESTATAIRES
- >
- recherche scientifique - instituts et laboratoires
Résultats pour
Recherche scientifique - instituts et laboratoires - Import export

EXTRACTIS
France
EXTRACTIS Innovation vous propose ses services à différents stades de votre projet de développement : de la preuve de concept (Stade Laboratoire), à la mise à l’échelle (Stade Micro-pilote), jusqu’à la validation et au transfert industriel (Stade Pilote) en vous garantissant la stricte confidentialité et la totalité de la propriété intellectuelle et industrielle. EXTRACTIS vous accompagne à l’échelle du laboratoire pour la mise au point de vos procédés ou encore dans la validation d’une preuve de concept afin de vous conforter dans les choix technologiques à mettre en œuvre ultérieurement. Doté d’un parc analytique performant, EXTRACTIS assure le suivi nécessaire au développement de vos procédés d’extraction et les contrôles analytiques de vos productions : • en chromatographie (GC, HPLC, HPIC); • avec des analyses physico-chimiques (texturométrie, viscosimétrie, rhéologie, chromamétrie…).
Demander un devis
EXTRACTIS
France
EXTRACTIS Innovation vous accompagne dans la conception et la réalisation de nouveaux produits/procédés de transformation de la biomasse végétale. Son équipe pluridisciplinaire et expérimentée vous propose des stratégies de fractionnement originales sur tout type de matière première végétale, co-produits ou fractions, l’évaluation de technologies innovantes à travers une connaissance approfondie du végétal.
Demander un devis
PROGENUS
Belgique
Contribuer au bien-être animal est un des engagements de Progenus. Nous avons à cœur de vous recommander les analyses génétiques propre à la race de votre animal. Il vous est également possible, via un profil ADN, de certifier son identité ainsi que sa filiation. Toutes ces analyses suivent la norme ISO17025 pour laquelle nous sommes accrédités. Nous sommes également certifiés ISAG 2006 avec un résultat au rings test en catégorie 1 (plus de 99% de fiabilité). En plus des maladies, vous pouvez effectuer d’autres tests afin de déterminer des marqueurs tels que la couleur de robe, la longueur des poils, la queue courte… Nous veillons au développement permanent de notre offre de tests en suivant quotidiennement les avancées du monde scientifique ou en y participant nous-même via notre département de Recherche. Vous avez besoin d’un test en particulier ou vous souhaitez avoir notre avis scientifique, nous sommes à votre écoute pour répondre à toutes vos questions.
Demander un devis
PROGENUS
Belgique
Nos services pour la recherche : - Services liés à l’ADN Développement de tests de PCR en temps réel, en simplex ou multiplex. Développement de méthodes d’extraction d’ADN pour tout type de matrices. Production de kits PCR (en tubes ou en plaques préremplies) selon la norme ISO13485. Développement de tests de LAMP (amplification ADN isotherme). Développement de PCR pour des applications de séquençage (Sanger et next generation sequencing NGS). Développement de tests d’analyse de fragments (analyse de fingerprint). - Services liés aux protéines Développement de tests ELISA. - Services liés aux pathogènes Développement de méthodes PCR pour la détection de virus et bactéries. Développement de lyses rapides sur bactéries et virus.
Demander un devis
EXTRACTIS
France
EXTRACTIS vous accompagne à l’échelle du laboratoire pour la mise au point de vos procédés ou encore dans la validation d’une preuve de concept afin de vous conforter dans les choix technologiques à mettre en œuvre ultérieurement. Avec ses 250m² de laboratoires, EXTRACTIS dispose de tous les moyens nécessaires à l’extraction, la purification et la modification de la biomasse végétale. Doté d’un parc analytique performant, EXTRACTIS assure le suivi nécessaire au développement de vos procédés d’extraction et les contrôles analytiques de vos productions : en chromatographie (GC, HPLC, HPIC); avec des analyses physico-chimiques (texturométrie, viscosimétrie, rhéologie, chromamétrie…).
Demander un devis
EXTRACTIS
France
EXTRACTIS Innovation vous accompagne dans la conception et la réalisation de nouveaux produits/procédés de transformation de la biomasse végétale. Son équipe pluridisciplinaire et expérimentée vous propose des stratégies de fractionnement originales sur tout type de matière première végétale, co-produits ou fractions, l’évaluation de technologies innovantes à travers une connaissance approfondie du végétal. Agrément Crédit Impôt Recherche (CIR) avec doublement de l’assiette Agrément Organisme de formation (N°22.80.00.772.80) LABELS ET CERTIFICATIONS CRT (Centre de Ressources Technologiques) qualifié par le Ministère de la Recherche depuis 1997 SRC (Structure de Recherche sous Contrat) par la BPI depuis 2000 Qualification ITAI (Institut Technique Agro Industriel) par le Ministère de l’Agriculture depuis 2007
Demander un devis
EAUX DE MERS - FOURNISSEUR EAU DE MER
France
Le plasma d’eau de mer Hypertonique est un complexe physiologique permettant le rééquilibrage ionique des liquides qui baignent les cellules de notre organisme. Le plasma d'eau de mer Hypertonique est un draineur cellulaire, l'eau de mer permet de stimuler le métabolisme digestif. Le plasma marin agit au niveau du plasma sanguin et sur les liquides extra cellulaires (hors de la cellule), son rôle est de maintenir les constantes de l’homéostasie et soutenir l’organisme face aux carences et les déséquilibres dû à certains modes de vie nepouvant couvrir les apports conseillés Un processus scientifique d’analyse en mer et ensuite de filtration successive à froid et microfiltration à froid 0,22µ garantissent une eau de mer vivante, fraîche et prébiotique de très haute qualité
Demander un devis
EAUX DE MERS - FOURNISSEUR EAU DE MER
France
Acteur de vos domaines: Sourcing matiére premiére Cosmetic Santé et bien etre Le magnésium marin sous forme sèche en poudre peut s’utiliser en complémentation alimentaire pour pallier aux carences et lutter contre le stress et la fatigue. L’oxyde de magnésium marin est extrait de notre eau de mer. Sa grande pureté garantie sa forte teneur en magnésium. L’eau de mer est deposée dans des marais salants pour obtenir une évaporation naturelle et concentrer l’eau en minéraux. Cette eau est ensuite plasée dans une tour d’atomisation afin d'evaporer l'eau et pour ne garder que le concentré minéral. La teneur de notre concentré minéral est à hauteur de 85 %.
Demander un devis
EAUX DE MERS - FOURNISSEUR EAU DE MER
France
Le plasma d’eau de mer Hypertonique est un complexe physiologique permettant le rééquilibrage ionique des liquides qui baignent les cellules de notre organisme. Le plasma d'eau de mer Hypertonique est un draineur cellulaire, l'eau de mer permet de stimuler le métabolisme digestif. Le plasma marin agit au niveau du plasma sanguin et sur les liquides extra cellulaires (hors de la cellule), son rôle est de maintenir les constantes de l’homéostasie et soutenir l’organisme face aux carences et les déséquilibres dû à certains modes de vie ne pouvant couvrir les apports conseillés. Un processus scientifique d’analyse en mer et ensuite de filtration successive à froid et microfiltration à froid 0,22µ garantissent une eau de mer vivante, fraîche et prébiotique de très haute qualité.
Demander un devis
EAUX DE MERS - FOURNISSEUR EAU DE MER
France
Le plasma d'eau de mer Isotonique est un complexe physiologique permettant la régénération du plasma sanguin et des liquides extracellulaires. L'isotonique à la même concentration en minéraux et oligo-éléments que le plasma humain : 9 gr / Litre. Forme identique au liquide qui baigne nos cellules. Le Plasma Isotonique est obtenu par une dilution de l’eau de mer intégrale avec de l’eau de source (eau de source peu minéralisée) afin d’obtenir une concentration qui correspond à la concentration physiologique identique des liquides du corps humain. Le plasma Isotonique «1 volume d’eau de mer Hypertonique (36g) dilué avec 3 volumes d’eau de source» est un plasma physiologique. Il favorise le renouvellement de l’ensemble des liquides du corps tel que le sang, la lymphe, les liquides extracellulaires et le liquide céphalo-rachidien.
Demander un devis
EAUX DE MERS - FOURNISSEUR EAU DE MER
France
Le plasma d’eau de mer Hypertonique est un complexe physiologique permettant le rééquilibrage ionique des liquides qui baignent les cellules de notre organisme Le plasma d'eau de mer Hypertonique est un draineur cellulaire, l'eau de mer permet de stimuler le métabolisme digestif Le plasma marin agit au niveau du plasma sanguin et sur les liquides extra cellulaires (hors de la cellule), son rôle est de maintenir les constantes de l’homéostasie et soutenir l’organisme face aux carences et les déséquilibres dû à certains modes de vie ne pouvant couvrir les apports conseillés. Un processus scientifique d’analyse en mer et ensuite de filtration successive à froid et microfiltration à froid 0,22µ garantissent une eau de mer vivante, fraîche et prébiotique de très haute qualité.
Demander un devis
EAUX DE MERS - FOURNISSEUR EAU DE MER
France
Le plasma d’eau de mer Hypertonique est un complexe physiologique permettant le rééquilibrage ionique des liquides qui baignent les cellules de notre organisme. Le plasma d'eau de mer Hypertonique est un draineur cellulaire, l'eau de mer permet de stimuler le métabolisme digestif. Le plasma marin agit au niveau du plasma sanguin et sur les liquides extra cellulaires (hors de la cellule), son rôle est de maintenir les constantes de l’homéostasie et soutenir l’organisme face aux carences et les déséquilibres dû à certains modes de vie ne pouvant couvrir les apports conseillés. Un processus scientifique d’analyse en mer et ensuite de filtration successive à froid et microfiltration à froid 0,22µ garantissent une eau de mer vivante, fraîche et prébiotique de très haute qualité.
Demander un devis
EAUX DE MERS - FOURNISSEUR EAU DE MER
France
Le plasma d'eau de mer Isotonique est un complexe physiologique permettant la régénération du plasma sanguin et des liquidesextracellulaires. L'isotonique à la mêm concentration en minéraux et oligo-éléments que le plasma humain : 9 gr / Litre. Forme identique au liquide qui baigne nos cellules. Le Plasma Isotonique est obtenu par une dilution de l’eau de mer intégrale avec de l’eau de source (eau de source peu minéralisée) afin d’obtenir une concentration qui correspond à la concentration physiologique identique des liquides du corps humain. Le plasma Isotonique «1 volume d’eau de mer Hypertonique (36g) dilué avec 3 volumes d’eau de source» est un plasma physiologique. Il favorise le renouvellement de l’ensemble des liquides du corps tel que le sang, la lymphe, les liquides extracellulaires et le liquide céphalo-rachidien.
Demander un devis
EAUX DE MERS - FOURNISSEUR EAU DE MER
France
Le plasma d’eau de mer Hypertonique est un complexe physiologique permettant le rééquilibrage ionique des liquides qui baignent les cellules de notre organisme. Le plasma d'eau de mer Hypertonique est un draineur cellulaire, l'eau de mer permet de stimuler le métabolisme digestif. Le plasma marin agit au niveau du plasma sanguin et sur les liquides extra cellulaires (hors de la cellule), son rôle est de maintenir les constantes de l’homéostasie et soutenir l’organisme face aux carences et les déséquilibres dû à certains modes de vie ne pouvant couvrir les apports conseillés. Un processus scientifique d’analyse en mer et ensuite de filtration successive à froid et microfiltration à froid 0,22µ garantissent une eau de mer vivante, fraîche et prébiotique de très haute qualité.
Demander un devis
EAUX DE MERS - FOURNISSEUR EAU DE MER
France
Le plasma d'eau de mer Isotonique est un complexe physiologique permettant la régénération du plasma sanguin et des liquidesextracellulaires. L'isotonique à la mêm concentration en minéraux et oligo-éléments que le plasma humain : 9 gr / Litre. Forme identique au liquide qui baigne nos cellules. Le Plasma Isotonique est obtenu par une dilution de l’eau de mer intégrale avec de l’eau de source (eau de source peu minéralisée) afin d’obtenir une concentration qui correspond à la concentration physiologique identique des liquides du corps humain. Le plasma Isotonique «1 volume d’eau de mer Hypertonique (36g) dilué avec 3 volumes d’eau de source» est un plasma physiologique. Il favorise le renouvellement de l’ensemble des liquides du corps tel que le sang, la lymphe, les liquides extracellulaires et le liquide céphalo-rachidien.
Demander un devis
EAUX DE MERS - FOURNISSEUR EAU DE MER
France
Le plasma d'eau de mer Isotonique est un complexe physiologique permettant la régénération du plasma sanguin et des liquides extracellulaires. L'isotonique à la même concentration en minéraux et oligo-éléments que le plasma humain : 9 gr / Litre. Forme identique au liquide qui baigne nos cellules. Le Plasma Isotonique est obtenu par une dilution de l’eau de mer intégrale avec de l’eau de source (eau de source peu minéralisée) afin d’obtenir une concentration qui correspond à la concentration physiologique identique des liquides du corps humain. Le plasma Isotonique «1 volume d’eau de mer Hypertonique (36g) dilué avec 3 volumes d’eau de source» est un plasma physiologique. Il favorise le renouvellement de l’ensemble des liquides du corps tel que le sang, la lymphe, les liquides extracellulaires et le liquide céphalo-rachidien.
Demander un devisVous vendez ou fabriquez des produits similaires ?
Inscrivez-vous sur europages et référencez vos produits

EAUX DE MERS - FOURNISSEUR EAU DE MER
France
Acteur de vos domaines: Agro-alimentaire Agriculture et Aquaculture Sourcing matiére premiére Cosmetic Santé et bien etre Thalassothérapie Nos services à votre disposition: Une eau de mer pélévée en pleine mer par notre bateau (micro-filtrée jusqu’à 0,22 microns) Elle contient 98 minéraux et oligo-éléments nécessaires à notre équilibre, dans des proportions proches du plasma sanguin et donc de nos besoins physiologiques Un conditionnement adapté à votre besoin (poches à usage unique stériles, IBC, …) Des productions d’eau de mer concentrée avec ou sans filtration Nos moyens d'exploitation: Analyse de l'eau avant prélevement sur notre navire, adaptabilité du lieu de prélévement et de la profondeur en fonction du milieu marin au jour de captation Pompe de captage d’eau mer anticorrosive
Demander un devis
DELPHI GENETICS
Belgique
GMP grade plasmid DNA production is based on robust, single-use, TSE-BSE-free methods and occurs in our GMP suite, at a maximum scale of 50L. Delphi Genetics offers one-stop-shop CDMO service for plasmid DNA GMP supply, including: GMP compliant Process and QC Development GMP E.coli cell banking (Master and Working Cell Banks) GMP DNA Manufacturing using TSE-BSE free and Single-use process, based on QP approved Master Batch Record QC and Release by Qualified Person (QP) Full Quality Control complying with the European pharmacopea 5.14 will be performed: Content and purity by UV measurement (260/280 nm) Visual inspection Identity by restriction and DNA sequencing (full plasmid sequencing) Safety tests: Endotoxins, Bioburden/Sterility and Mycoplasma Residual RNA content Residual host protein Residual host genomic by Q-QPCR pH Topology Other tests can be performed on request. QP Released GMP Plasmid DNA is provided with TSE-BSE-free certificate and Certificate of Analysis.
Demander un devis
DELPHI GENETICS
Belgique
Delphi Genetics provides customized plasmid DNA production services, from milligram to gram scale, for your R&D and clinical activities. We take care of your plasmid DNA material for viral vectors production and naked DNA-based gene therapy. Our service includes the supply of: R&D grade for non-clinical use, support for lead identification, clone screening, STABY evaluation. High Quality and GMP grade plasmid DNA for viral and non-viral (pre-)clinical activities.
Demander un devis
DELPHI GENETICS
Belgique
For manufacturing recombinant proteins or DNA, plasmid instability is a significant concern. Typical biomanufacturing processes in prokaryotes require the use of bacterial plasmids as vectors carrying the gene of interest to be over-expressed. It has been demonstrated that the growth of plasmid bearing cells is significantly reduced relative to plasmid free hosts, simply because protein production represents a significant burden on cellular metabolism. This is of significant concern in the Biopharmaceutical Industry as both the yield and the production reproducibility of recombinant molecules are significantly lowered by that fact. Antibiotic resistance genes are the most common selectable markers used in fermentation processes to avoid plasmid free cells to overgrow the culture. However antibiotics are expensive compounds and they (or their degradation products) can contaminate the biomass or production product.
Demander un devis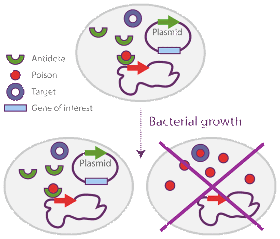
DELPHI GENETICS
Belgique
Several alternative strategies have been developed to minimize the risk that plasmid-free cells overtake the culture. One of the most promising of those strategies relies on the use of postsegretional killing genes (so called poison / antidote genes or selection modules) that induce host killing upon plasmid loss. Delphi Genetics has designed a novel and highly effective stabilization system, called Staby®, based on the use of selection modules naturally found in plasmids, bacterial chromosomes and bacteriophages. A selection module (like the ccd system used by Delphi Genetics) is typically organized as an operon and composed of two genes: the ccdB selection gene codes for a small stable protein which is toxic for E. coli, whereas the ccdA antidote gene codes for a small unstable protein that neutralizes the toxic protein both transcriptionaly and via protein-protein interactions. This unique system allows for the perfect stabilization of the plasmid without the use of antibiotics
Demander un devis
DELPHI GENETICS
Belgique
With thousands of DNA vectors designed and constructed, Delphi Genetics is a Partner of choice to generate your plasmid of interest. Size does not matter, that's the strategy of cloning that drives the success of a plasmid construction. We perform the construction of your vector from strategy design to final plasmid production. We work with standard plasmid DNA as well as with large constructs, such as BAC and Cosmid. Our plasmid DNA construction service includes: Sequences analysis Design and strategy Gene Synthesis Cloning of PCR fragment DNA fragment assembling Site directed mutagenesis DNA sequencing Plasmid productionA new hope for Covid-19 treatment Delphi Genetics more than triples its production capacity and receives GMP certification. New Plasmid DNA production capacities now immediately available. Delphi to attend Phacilitate Leaders World in Miami, Jan 21-24 Delphi Genetics significantly expands its capacities for cGMP plasmid DNA GMP Certification for Plasmid DNA
Demander un devis
DELPHI GENETICS
Belgique
In order to maximize protein expression, the Staby® system was combined to the proven T7 polymerase technology for the over-expression of recombinant proteins: the gene of interest is under the control of the T7 promoter. This combination is called StabyExpress®. The pStaby1 plasmid contains the gene encoding the antidote protein (ccdA) which counteracts the action of the ccdB protein. The SE1 strain, in which the ccdB selection gene is inserted in the chromosome in order to stabilize the pStaby1 plasmid has been engineered by Delphi Genetics as a dedicated bacterial strain for protein expression and is derived from BL21(DE3). Consequently, IPTG has to be used to induce protein expression, an alternative strategy being the use of an auto-inducible medium (such as Delphi Genetics' Staby®Switch medium). Staby®Express ensures that only plasmid-bearing clones can grow whereas every clone losing the plasmid will inevitably die.
Demander un devis
DELPHI GENETICS
Belgique
Delphi Genetics offers the benefits of its proprietary Staby® technology through partnering opportunities. We intend to build high-value strategic collaborations for exploring the benefits of the Staby® technology in various DNA & Protein bioproduction settings. Partnering is a key component of our service delivery strategy. Delphi Genetics is open to consider any collaborative projects at all stages of development from R&D to the commercialization of your bioproduct. We seek collaborative partnerships with Biotech & Pharma companies that could enable us to contribute to your development on either a risk-sharing or out-licensing basis. In order to consolidate a long term relationship with its partners, Delphi Genetics is currently developing a brand new GMP production facility. This mid-scale capacity facility will join technology and flexibility to provide plasmid DNA and protein with high value for your clinical phases.
Demander un devis
DELPHI GENETICS
Belgique
All E. coli strains can be adapted to the Staby® technology. This adaptation is linked to the insertion into the bacterial chromosome of the CcdB toxic gene. Based on our strong knowledge of E. coli genetic and our know-how in genetic engineering we can easily adapt the strain of your choice and genetically modify it for the Staby® technology. On the same basis, we can genetically modify the strain of your choice to introduce mutation, deletion or to restore activity of certain gene. We can also introduce new gene to give new characteristic to your strain. One example would be to allow your strain of interest to grow and use new or unusual substract. All these modifications are finalized without any genetic trace
Demander un devis
DELPHI GENETICS
Belgique
We are continuously improving and developing our Staby® technology to provide new adaptation proposing the best solution to our Customer. The Staby® technology has been adaptated in different fields and can be proposed on a tailor made solution basis or on a service basis. The Staby® technology applications actually available are the following. Feel free to contact us for other request
Demander un devis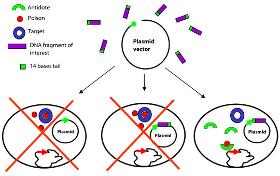
DELPHI GENETICS
Belgique
Traditionally clone selection was based on the disruption of a genetic marker (e.g. blue and white screening based on the disruption of the LacZ gene), plasmid retention was based on antibiotic resistance genes. The development of vectors employing selection modules circumvents these issues and permits growth of bacteria harbouring insert-bearing plasmids only. Typically, the vectors used in these systems express a gene product that is lethal to certain bacterial hosts. The lethal gene is inactivated by insertion of a segment of foreign DNA and therefore, toxicity is relieved. The most efficient technical solution remains the killing of bacteria harbouring an insertless vector, or the selection of bacteria harbouring the recombinant vector, the so-called positive selection. The ccdB technology developed by Delphi Genetics has proven to be a successful approach for constructing positive selection vectors
Demander un devis
DELPHI GENETICS
Belgique
Because the stabilization relies on the presence of only two genetic elements, one on the plasmid the other in the expression strain, the stabilization technology is readily adaptable into any existing E.Coli based expression platform. Delphi Genetics can customize any vectors and strains by adding the stabilization cassette into the vector and the selection gene into the chromosome of the chosen expression strain. Furthermore Delphi Genetics has developed a comprehensive licensing policy for those who would wish to integrate the stabilization system into an existing platform.
Demander un devisRésultats pour
Recherche scientifique - instituts et laboratoires - Import exportNombre de résultats
34 ProduitsPays
Type d'entreprise
